Chemical reduction of CO2
A group of chemists around Professor Mougel is investigating the electrochemical reduction of CO2 into alcohol and related molecules. The resulting alcohol and related hydrocarbons could be used as chemical feedstocks while removing CO2 from the air. Much of what happens in this promising reaction remains obscure, but Professor Mougel and his team are shedding light on what is going on.

Victor Mougel's group has recently joined forces with Toyota to study this reaction in more detail. We talked to Prof. Victor Mougel and Coral Felipe (R&D Engineer at Toyota’s Material Engineering Division).
Prof. Mougel, where do you envision the primary applications for your CO2 reduction research?
Victor Mougel: I believe its significance lies in providing chemical feedstock. As humanity strives to decarbonise and curb greenhouse gas emissions, this reaction offers the potential to sustain the use of chemicals — essential for various sectors like materials, medicine, fertilizers, and more. Additionally, it can facilitate energy storage in chemical form, surpassing battery efficiency. Furthermore, it enables carbon-neutral fuel production, particularly crucial in sectors where a complete transition to electric vehicles is not feasible.
How did this collaboration come about?
Victor Mougel: Toyota approached me at a conference and expressed interest in collaboration. After discussing and setting joint objectives, we established a one-year project, with potential for future expansion.
Coral Felipe: At Toyota, we searched Europe for collaborators and found Prof. Mougel’s group. Their expertise in mimicking nature’s processes to convert CO2 to hydrocarbons complements ours, making collaboration ideal. Their groundbreaking research on scalable CO2 reduction aligns with Toyota's commitment to sustainability, driving us closer to our goal of a more sustainable future.
How is the collaboration going?
Victor Mougel: Our collaboration is strong, benefiting from valuable feedback from Toyota. We value insights from outside academia and are keen to integrate industrial perspectives into our research. Both our team and Toyota's R&D are eager to explore new materials and technologies, advancing towards a carbon-neutral society.
Coral Felipe: In this partnership, Toyota guides the project, setting direction and goals, while Prof. Mougel's team offers technical insights and innovative approaches. Their expertise in bio-inspired solutions enables the exploration of new avenues and the application of cutting-edge research. Transparency, communication, and mutual respect characterize our collaboration, as both teams work harmoniously towards shared objectives.
What are Toyota's main objectives for the joint project?
Coral Felipe: Our collaboration with Prof. Mougel's group is pivotal for Toyota Motor Europe’s research initiative. We explore bio-inspired CO2 reduction to unlock innovative pathways for converting CO2 into hydrocarbons, aligning with our environmental objectives (e.g., carbon neutrality by 2040). Although in the early stages, we aim to develop viable solutions that can be implemented within our operations and potentially commercialised.
CO2 is probably the least wanted molecule today, especially when it is emitted into the atmosphere. Reducing it to alcohol and related chemicals through a chemical reaction would seem to be very welcome for several industries. Why is this process not yet widely available?
Victor Mougel: The chemical reaction is complex, yielding hydrocarbons along with other intermediates and by-products that compromise its efficiency and obscure its mechanisms. To clarify the reaction, we investigate various systems focusing on three key factors: the three-phase interface, local pH, and surface effects of the solid catalyst. Spectroscopic methods enable molecular-level insights, facilitating accurate analysis. Additionally, we design and develop catalysts, predominantly copper-based, although the stability of many requires improvement to realise their industrial potential.
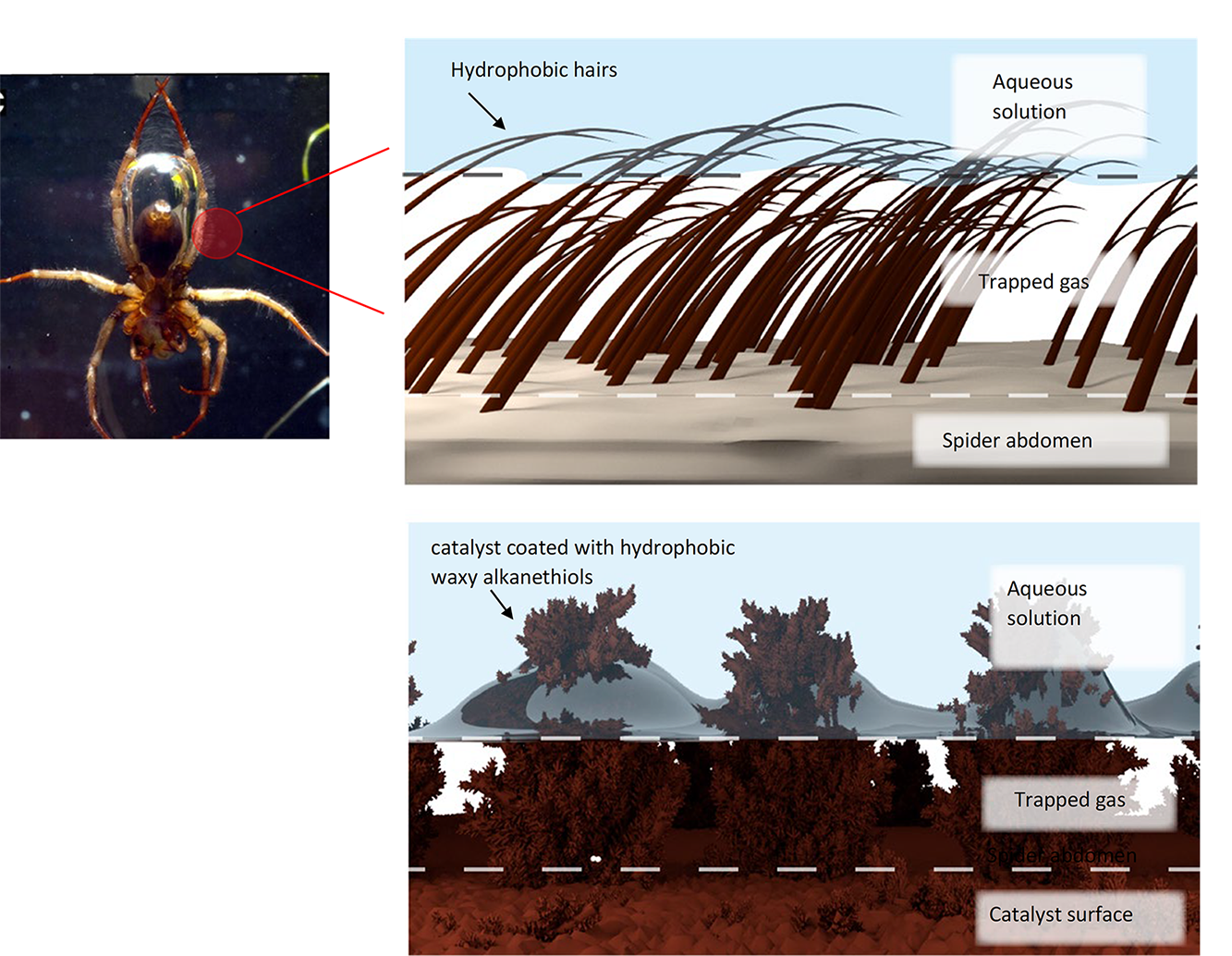
Prof. Mougel, your research is often inspired by nature, such as a diving spider that breathes underwater by maintaining a layer of gas around itself. How did this improve the reaction?
Victor Mougel: The diving spider's superhydrophobic layer allows it to breathe underwater by trapping air. By replicating this concept with waxy alkanethiols on our catalysts, we achieved higher hydrocarbon output by confining carbon gas near the catalyst. Drawing inspiration from nature, we leverage billions of years of evolution to enhance reaction efficiency. Without this spider-inspired system, CO2 struggles to reach the electrode, resulting in low hydrocarbon output. Additionally, this research may lead to electrochemically reducing N2 to ammonia, offering a CO2-neutral alternative to the Haber-Bosch process, a significant industrial advancement.
Contact/Links:
Mougel Group - Bioinspired Molecules and Materials
Do you want to get more "News for Industry" stories?
external page Follow us on LinkedIn
Are you looking for research partners at ETH Zurich?
Contact ETH Industry Relations

